![]() Introduction
to Protein Structure
Introduction
to Protein Structure
Amino Acids
Proteins are polymers of a-amino acids.
There are twenty amino acids that are commonly found in proteins. Each amino acid has a similar, yet unique structure (Figure 1).
Figure1: 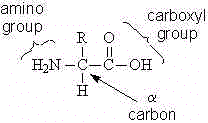
All of the amino acids (except for proline) have a carboxyl group and an amino group. At physiological pH the natural amino acids exist as zwitterions, with a negatively charged carboxyl group and a positively charged amino group.
The a carbon of most of the amino acids is chiral. Thus there are two stereoisomers of most of the amino acids.
Each amino acid has a different side chain (or R group). The side chains vary greatly in their complexity and properties. The side chain of glycine is simply a hydrogen. The side chain of tryptophan is based on the aromatic, bicyclic indole group. Amino acids are classified by the chemical nature of their side chains. One useful classification of the amino acids divides them into two groups, the polar (or hydrophilic) amino acids have side chains that interact with water, while those of the nonpolar (or hydrophobic) amino acids do not.
The non-polar amino acids include: alanine, cysteine, glycine, isoleucine, leucine, methionine, phenylalanine, proline, tryptophan, tyrosine and valine.
The polar amino acids include: arginine, asparagine, aspartic acid (or aspartate), glutamine, glutamic acid (or glutamate), histidine, lysine, serine, and threonine.
Another subgroup of the amino acids are those with ionizable side chains. These include: aspartate, glutamate, histidine, cysteine, lysine, tyrosine and arginine. These amino acids contribute to the charge exhibited by peptides and proteins
Detailed data (including pKa's, parameters for structure prediction and miscellaneous properties) can be found at a web site maintained by Charles S. Gasser at the University of California, Davis.
Copyright © 1998, 1999, 2007 by Frank R. Gorga; Page maintained by F.R. Gorga; Last updated: 12-Mar-2007