
The molecular structure of molecules may be predicted with surprising accuracy using VSEPR concepts. The chief tenet of the theory is that electron groups (either lone pairs or bonding pairs of electrons) will try to maximize the distance between themselves and minimize repulsions between electron pairs. Doing so leads to the formation of 5 primary electron group geometries which the molecular structures of the molecules are based on.
Steps for predicting the molecular geometry about a particular atom. (to go directly to the models/examples, click here)
(1) Determine the best Lewis Structure
(2) Identify the number of electron groups present around a particular atom
(3) Establish the "Electron Group Geometry" based on the number of electron groups
This geometry is the the lowest energy arrangement of the electron groups that minimizes electron pair repulsions
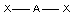
"linear" 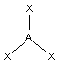
"trigonal planar" 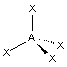
"tetrahedral" 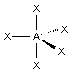
"trigonal bipyramidal" 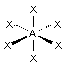
"octahedral"
(4) Place lone pair electrons in positions that minimize the # of 90° lone pair-lone pair interactions
For trigonal bipyramidal geometry, the lone pairs always occupy the equatorial positions
(5) Place atoms (bonding pairs of electrons) in remaining positions
(6) Assign the Molecular geometry (shape of the molecule) by describing the location of the atoms.
(7) Note any distortions from the ideal bond angles.
Lone pairs require more room and will force atoms (bonding pairs) closer together than expected based upon the electron group geometry
For examples of each molecular geometry
and how it is formed,
click on the appropriate # of electron group
|
Number of Electron groups
|
| Bridgewater Homepage | Chemistry Homepage | Search Bridgewater | Search Chemistry Pages |
