Periodic Trends
The
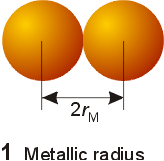
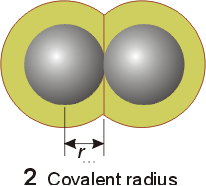
definition of atomic radius will vary slightly with the system under question.
Typically metallic and covalent radius is refered to as the atomic radius.
Ionization Energy
- Energy required to completely remove one electron from a gaseous atom
- always endothermic, requires energy
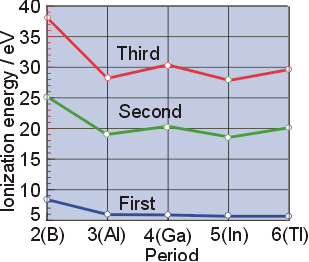
- successive ionizations are higher in energy
- increasing positive charge makes removing
next electron more difficult
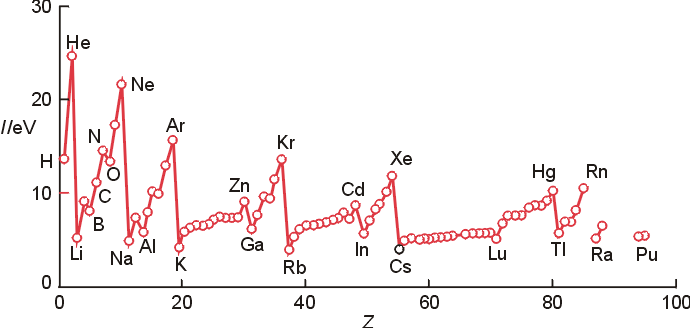
- General Trends
- IE generally increases left to right across
a period (some exceptions! see below)
- increaseing effective nuclear charge
(Zeff) makes removal more difficult
- IE generally decreases down a group
- valence electron is in higher energy
orbital that is further from the nucleus and therefore more easily
removed
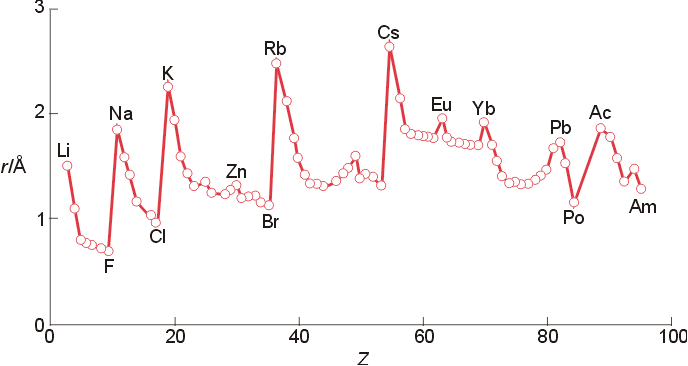
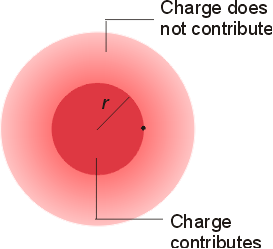
Atomic Radius:
- Increases down the group
- valence electrons placed in orbitals with
increasing n
- Decreases across (left to right) the period (esp. within s- and p-block)
- electrons placed in orbitals within same
level
- nuclear charge increases steadily since electrons
in same shell are unable to screen efficiently
-
Note the following trend of atomic radii for group 6 metals:
Cr 1.29 angs., Mo 1.40 angs, W 1.41 angs.
Is this unusual? What is occurring?
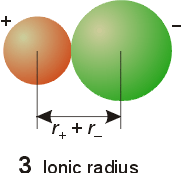
Ionic Radii
- Cations
- smaller radii than neutral, isoelectronic
atom
- As charge increases, the radius decreases
- increased nuclear charge has stronger
attraction for electron
- reduced electron-electron repulsions
- Anions
- radius increases with greater negative charge
- increased electron-electron repulsions
- Lanthanide contraction :
- second and third row transition metals have
nearly the same radii
- 4f orbitals are filled and provide poor
shielding
- Zeff increase offsets expected increase
in radii
Variations
in atomic radii through the periodic table.
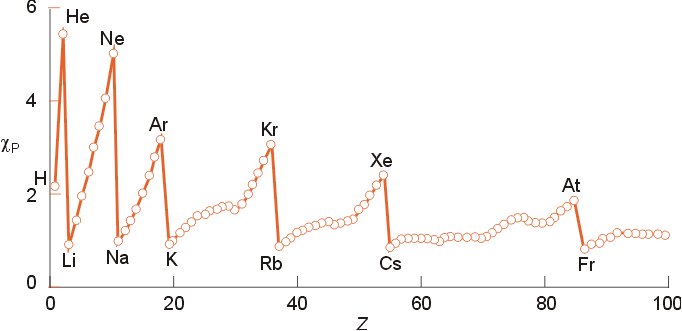
Electronegativity:
- X is the ability of a atom to attract electron density to itself within
a bond.
- Atoms with high electronegativities will
attract more electron density to itself
- electronegativities may be used to determine
bond polarity
- Atoms with low electronegativity are termed electropositive
- Electronegativity will increase with increasing oxidation state
- Several Electronegativity scales have been developed
- the best known is Pauling's scale based on
thermodynamic data
- Muliken's relates electronegativity to the
average of the ionization energy and electron affinity
- Trends in Electronegativity
- Increases left to right across a period
- Decreases down a group
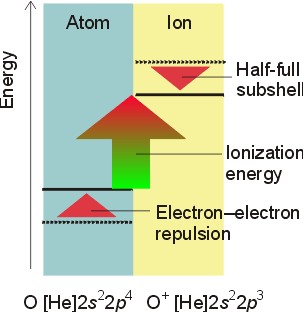
Electron Affinity
- Energy change that occurs when a gaseous negative ion releases an electron
- can be thought of as the ionization energy
of an anion.
- value may be positive or negative (endothermic
or exothermic)
- the more negative the value, the more favorable
the process
- Trends mimic that of ionization energy
- EA generally increases left to right across
a period (more negative and fav.)
- EA generally decreases down a group (less
negative and less favorable)