![]() Return
to Prof. Haefner's Chem 111 Homepage
Return
to Prof. Haefner's Chem 111 Homepage
A Hydrogen Bond is an unusually strong dipole-dipole interaction
It will only occur between a hydrogen atom attached (covalently bonded) to N, O or F and a lone pair of electrons on a neighboring N, O, or F.
![]()
Since the hydrogen-bond is weaker than a covalent bond, the atoms must be fairly close together
Given a set of molecules, you must be able to predict when and where a hydrogen bond will form
Why only with N, O, and F?
Examples of compounds with extensive hydrogen bonding interactions
Note that many compounds can exhibit hydrogen bonding
General Rule of Thumb ....
..... the greater the number of hydrogen bonds that can be formed the stronger the intermolecular forces
each hydrogen bond is worth 4 - 40 kcal/mol, (5-15 kcal/mol typical)
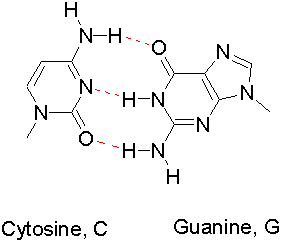
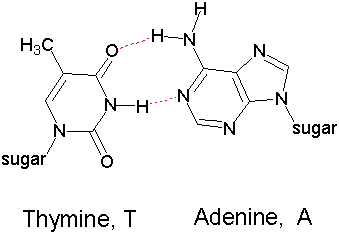
Hydrogen bonding is critical in biochemistry
protein folding (alpha helix, beta sheets)
molecular recognition (enzymatic catalysis, immune response etc.)
Double Helix formation
between two strands of DNA (Watson-Crick Base pairing)
 ....
....