
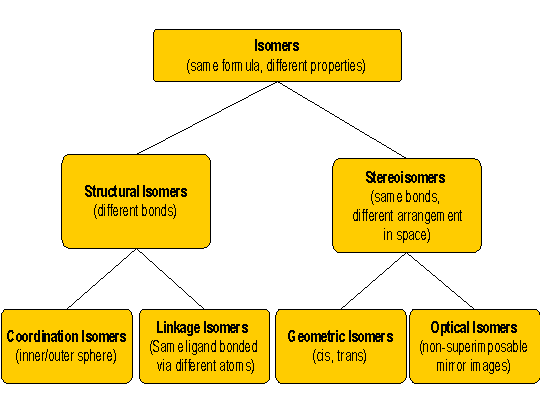
|
Coordination Isomer
|
form of Structural Isomer
|
Coordination isomers vary according to which anions are
coordinated to the metal (inner sphere) and which are acting as counterions
to the complex ion (outer sphere)
In one example the choloride is bound to the cobalt and bromide is the counter ion. In the other coordination isomer the bromide is coordinated to the cobalt and chloride is the counter ion |
|
Linkage Isomer
|
form of Structural Isomer
|
Linkage isomers contain the same ligands but one or more of the ligands are coordinated to the metal through a different atoms. These ligands are said to be ambidentate. A ligand that has lone pairs on two different atoms can potentially be ambidentate
In this example, nitrite ion NO2- is ambidentate and may be bound through either the lone pair on the central nitrogen atom or the lone pare on the oxygen. This gives rise to two linkage isomers. |
|
Geometric Isomer
|
form of Stereo-Isomer
|
Often refered to as cis/trans isomerism. The ligands are
coordinated through the same atoms but their spatial orientation varies.
Most common forms are:
|
|
Optical Isomer
|
form of Stereo-Isomer
|
In this case, the spatial orientation of ligands is such that the two isomers are non-super imposable mirror images of one another. There are two common occurences of this type of isomerism
Note that trans isomers cannot be optical isomers |
||||||||||||
