

Steven C. Haefner
Associate Professor of Chemistry
Bridgewater State College
B.S., 1987, University of California, Los Angeles
Ph. D., 1992, Michigan State University ( Kim R. Dunbar)
Joint Postdoctoral Research Associate, 1992 - 1996
Texas A&M University (F. Albert Cotton) and Los Alamos National Laboratory
(Alfred P. Sattelberger)
Assistant Professor of Chemistry, Wake Forest University, 1996 - 2000
Research Activities
My groups research interests lie in the areas of synthesis, spectroscopy and structures of transition metal inorganic and organometallic complexes and their subsequent applications toward small molecule chemistry, catalysis, molecular recognition and the development of supramolecular systems. We are particularly interested in understanding and exploiting cooperativity between metal atoms in polymetallic systems in order to effect chemical and physical processes.
Currently we are focusing our efforts on three diverse projects.
(I) Bimetallic Receptors for the Selective Recognition of Saccharides
Our laboratory is investigating the use of bimetallic cleft-shaped molecules in selective binding and recognition of oligosaccharides. Oligosaccharides, in the form of glycoconjugates, are involved in a diverse range of cell-cell phenomena including cell-cell adhesion, signaling, and recognition. Our goal is to develop metal-based oligosaccharide receptors that can effectively bind specific glycoconjugates. Such molecular species have the potential to inhibit cell-cell recognition events involved in tumor cell metastasis and inflammation . Our approach is to develop polymetallic molecular cleft molecules that may act as chelating agents towards carbohydrates by binding the sugar at multiple points. With these points in mind, we wish to answer the following fundamental questions; (1) Does pre-organization of the metal ions obtained by the use of cleft-shaped ancillary ligands provide the thermodynamic stabilization required for strong saccharide complexation? (2) can chemical selectivity for different saccharides be achieved based upon the relative disposition of the metal ions within the molecular cleft in conjunction with complimentary hydrophilic and hydrophobic interactions between the saccharide and the cleft?
We are examining the interactions of (2-hydroxybenzyl)iminodiacetic acid derived receptors with simple monosaccharides. These receptors are being prepared by covalently linking chloromethyl substituted (2-hydroxybenzyl) iminodiacetic acid groups together using a variety of spacer molecules (Scheme 1). The analogous N-(o-hydroxybenzyl)iminodiacetic acid
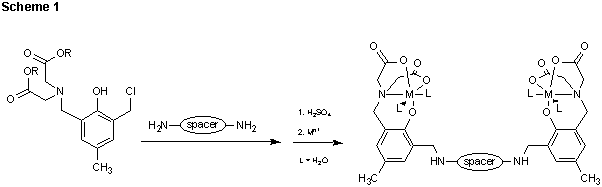
based ligands form tightly bound tetradentate complexes with a number of transition metals. The tetradentate coordination geometry of the HBDA ligands creates two accessible cis-coordination sites on each metal that are ideally suited for binding to a cis-diol group of a sugar. The preorganized binding sites thus created will eliminate the entropic barriers associated with dimerization of monometallic fragments and will greatly enhance the ability of these receptors to bind saccharides. The initial objective is to document the binding of common monosaccharides such as mannose, glucose and galactose within the HBDA derived molecular clefts. With this information we will be prepared to design larger more complex cleft species that will specifically target the sialylated and fucosylated oligosaccharides found at the terminus of glycoconjugates in biological systems.
(II) One Dimensional Metal-Metal Bonded Assemblies Supported by Hydrogen Bonded Networks
The objective of this project is the rational development of one-dimensional oligomeric and polymeric materials containing dimetal subunits directly linked to one another through metal-metal bonding interactions. Despite their many similarities with carbon-carbon multiple bonds, metal-metal multiple bonds do not undergo polymerization reactions ubiquitous among unsaturated hydrocarbons. The steric constraints imposed by the supporting ancillary ligands inhibit metal-metal bond association and prevent oligomerization from occurring. Our approach to this problem is to develop metal complexes that incorporate supporting ligands capable of engaging in hydrogen bonding interactions between dimetal subunits. To this end, we are investigating reactions between tetrabridged dimetal species containing unsubsituted amidinate ligands (hydrogen-bond donors, D) and carboxylate ligands (hydrogen-bond acceptors, A) (Scheme 2). We anticipate that a linear arrangement of
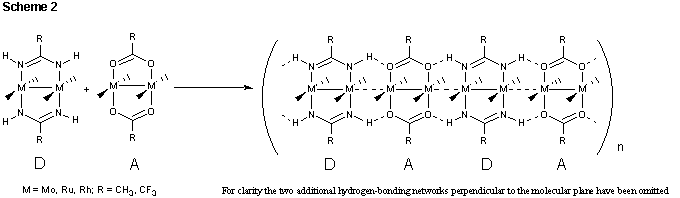
an alternating sequence of hydrogen bond donors, D, and hydrogen bond acceptors, A, will create set of four co-linear hydrogen bonding networks. The hydrogen-bonding network thus formed will be used to reinforce metal-metal interactions and act as a scaffold for the formation of linear metal chains. Elimination of steric constraints will allow direct investigation of the electronic requirements needed for metal-metal bond polymerization to occur.
The current study will provide new insight into the electronic factors required for metal-metal bond polymerization and establish a new reactivity paradigm for the metal-metal multiple bond. Furthermore, the success of the project may ultimately lead to new molecular architectures with unusual energy transport properties that will be useful in the development of nanoscale devices.
(III) Solvated Cations Possessing Metal-Metal Multiple Bonds.
Metal complexes supported by highly labile solvent molecules represent valuable precursors for the preparation of catalysts, novel solid state materials and other supramolecular constructs. In this regard we are interested in the use of N,N-dialkylformamides and N,N-dialkylacetamides to support metal-metal multiply-bonded compounds. We have successfully prepared and isolated the dinuclear molybdenum(II,II) cations [Mo2(DMF)8]4+ and [Mo2(DMA)8]4+. These species represent the first dinuclear homoleptic DMF and DMA complexes to be isolated. Oxidation of [Mo2(DMF)8]4+ was found to give the unusual solvated metal cluster [Mo3O4(DMF)9]4+. We are currently examining solvated DMF and DMA complexes of other dinuclear metal systems (e.g. Rh24+, Re24-6+ and W24+), as well as the use of other less conventional solvents such as hexamethylphosphoramide (HMPA) and tetramethyl urea (TMA).
Selected Publications
Spectroscopic and Structural investigation of [Mo2(DMA)8]4+, the First Homoleptic N,N-Dialkylamide Complex Possessing a Metal-Metal Bond. S. C. Haefner and C. S. Day, J. Am. Chem. Soc. manuscript in preparation.
Synthesis and Reactivity of Homoleptic Dimethylformamide Complexes Possessing a Metal-metal Multiple. S. C. Haefner, E. L. Gilmore, P. Hodes, S. M. Monnier and C. S. Day, Inorg. Chem. manuscript in preparation.
Synthesis and Structure of Pyridyl-Functionalized Amidines. S. C. Haefner, T. Coles, E. L. Gilmore, S. M. Monnier and C. S. Day, Polyhedron, manuscript in preparation.
Facile Hyrolysis of N,N'-Dipyridylformamidines using Divalent Metal Ions. S. C. Haefner, E. L. Gilmore, S. M. Monnier and C. S. Day, Inorg. Chim. Acta, manuscript in preparation.
Metal-Metal Multiply-Bonded Complexes of Technetium. 8. Structural and Spectroscopic Characterization of Alpha and Beta Isomers of Tc2Cl4(dppe)2. F. A. Cotton, S. C. Haefner and A. P. Sattelberger, Inorg. Chim. Acta, 1999, 288, 69.
Synthesis, Reactivity, and Structures
of (m-acetamidato)hexakis(acetonitrilo)dimolybdenum(II)
tris(tetrafluoroborate) and Derivatives. F. A. Cotton, L. M. Daniels,
S. C. Haefner and F. E. Kühn, Inorg. Chim. Acta, 1999, 287,
159.
Two DiphenylFormamidinato Compounds of the Quadruply-bonded Re26+
Core. F. A. Cotton, L. M. Daniels, S. C. Haefner Inorg. Chim. Acta,
1999, 285, 149
Metal-Metal Multiply-Bonded Complexes of Technetium. 7. Oxidative Decomposition of Tetrachlorotetrakis(dimethylphenylphosphino)ditechnetium(II) by an Aminium Hexachloro-antimonate(V). F. A. Cotton, S. C. Haefner and A. P. Sattelberger Inorg. Chim. Acta, 1998, 271, 187.
Metal-Metal Multiply-Bonded Complexes of Technetium. 6. Reductive
Cleavage of the Tc![]() Tc
Triple Bond in [Tc2CH3CN)10][BF4]4.
F. A. Cotton, S. C. Haefner and A. P. Sattelberger Inorg. Chim. Acta
1997, 266, 55.
Tc
Triple Bond in [Tc2CH3CN)10][BF4]4.
F. A. Cotton, S. C. Haefner and A. P. Sattelberger Inorg. Chim. Acta
1997, 266, 55.
Metal-Metal Multiply-Bonded Complexes of Technetium. 5. Tris- and Tetra-formamidinate Complexes of Ditechnetium. F. A. Cotton, S. C. Haefner and A. P. Sattelberger Inorg. Chem. 1996, 35, 7350.
Metal-Metal Multiply-Bonded Complexes of Technetium. 4. Photodissociation
of the Tc![]() Tc
Triple Bond in [Tc2CH3CN)10][BF4]4.
F. A. Cotton, S. C. Haefner and A. P. Sattelberger J. Am. Chem. Soc.
1996, 118, 5486.
Tc
Triple Bond in [Tc2CH3CN)10][BF4]4.
F. A. Cotton, S. C. Haefner and A. P. Sattelberger J. Am. Chem. Soc.
1996, 118, 5486.
Metal-Metal Multiply-Bonded Complexes of Technetium. 3. Preparation and Characterization of Phosphine Complexes of Technetium Possessing a Metal-Metal Bond Order of 3.5. F. A. Cotton, S. C. Haefner and A. P. Sattelberger Inorg. Chem. 1996, 35, 1831.
Metal-Metal Multiply-Bonded Complexes of Technetium. 2. Preparation and Characterization of the Fully Solvated Ditechnetium Cation [Tc2(CH3CN)10]4+. J. C. Bryan, F. A. Cotton, L. M. Daniels, S. C. Haefner and A. P. Sattelberger Inorg. Chem. 1995, 34, 1875.
Metal-Metal Multiply-Bonded Complexes of Technetium. 1. Synthesis and Structural Characterization of the First Phosphine Complexes that Contain a Tc-Tc Multiple Bond. C. J. Burns, A. K. Burrell, F. A. Cotton, S. C. Haefner and A. P. Sattelberger Inorg. Chem. 1994, 33, 2257.
Carbon Monoxide Reactions of the Fluxional Phosphine Complex
(![]() 3-PR3)Mo(CO)3
(R = 2,4,6-trimethoxyphenyl). K. R. Dunbar, J.-S. Sun, S. C. Haefner, and J.
H. Matonic Organometallics, 1994, 13, 2713.
3-PR3)Mo(CO)3
(R = 2,4,6-trimethoxyphenyl). K. R. Dunbar, J.-S. Sun, S. C. Haefner, and J.
H. Matonic Organometallics, 1994, 13, 2713.
Crystallographic Disorder in the Orthorhombic Form of RhCl(CO)(PPh3)2: Relevance to the Reported Structure of the Paramagnetic Impurity in Wilkinson's Catalyst. K. R. Dunbar and S. C. Haefner Inorg. Chem. 1992, 31, 3676.
Reversible Carbon Monoxide Addition to Sol-Gel Derived Composite Films Containing a Cationic Rhodium (I) Complex: Toward the Development of a New Class of Molecule-Based CO Sensors. J. I. Dulebohn, S. C. Haefner, K. A. Berglund and K. R. Dunbar Chem. Mater. 1992, 4, 506.
Structural and Spectroscopic Characterization of a Paramagnetic Rh(II) Complex With Isocyanide and Phosphine Ligands. K. R. Dunbar and S. C. Haefner Organometallics 1992, 11, 1431.
Reversible Carbon Monoxide Binding to Cation Complexes of Rh(I) and Rh(II). K. R. Dunbar, S. C. Haefner, and C. Bender J. Am. Chem. Soc. 1991, 113, 9540.
| Bridgewater Homepage | Chemistry Homepage | Search Bridgewater | Search Chemistry Pages |
