Incorporating three dimensional information into line structures.
| All of the lines (bonds) that we have used so far in drawing structures have been "normal" lines. These normal lines are used to represent bonds that lies in the plane of the drawing surface (i.e the computer screen, the paper, the chalkboard, etc.) In order to represent bonds projecting out of this plane, we use "dashed" and "wedged" bonds. | ||
|
||
|
||
| The three common types of bonds used in drawing chemical structures: | ||
|
||
Thus, we can take a simple
molecule such as methane and represent its tetrahedryl geometry using wedged and dashed
bonds as follows: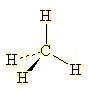 |
||
| Compare this to the molecular model: | ||
| We can also use this convention to represent stereoisomers. | ||
| Take a look at the two stereoisomers of 2-bromobutane, which we looked at before. | ||
|
||
(S)-2-bromobutane |
(R)-2-bromobutane |
|
|
|
|
|
|
|
|
|
|
|
||
Copyright © 1996 -1999, 2007 by Frank R. Gorga - All rights reserved.
Last Update: 12-Mar-2007