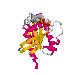 Ras Changes Conformation upon "Switching"
Ras Changes Conformation upon "Switching"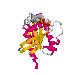 Ras Changes Conformation upon "Switching"
Ras Changes Conformation upon "Switching"
The two forms of ras (i.e. ras·GTP and ras·GDP) have different conformations. If one looks at the locations of the alpha carbons of each residue in both structures you find that, although there are small changes in conformation throughout the ras molecule, two small sections show major changes in structure as the molecule switches from the GDP complex to the GTP complex (or vice versa). These section are called the "switch regions". Each switch region comprises about eight amino acids. Thus, only about ten percent of the protein (22 out of about 170 residues total) undergoes major structural changes upon switching from "off" to "on".
Use the CHIME model shown below to explore this conformational switch:
| Highlight switch regions (toggle on/off) | |
|
|
| Switch Conformations (toggle
between GTP & GDP complexes) . |
|
|
|
|
|
|
Carefully examine the spacefilled model to see how the surface of the protein changes. It is the differences in this surface that cause effector molecules to bind to the ras·GTP complex but not the ras·GDP complex.
Examine the cartoon model to see how the secondary structure changes. Notice how residues 64-74 (at one end of the "orange group" of amino acids) form an alpha helix in the ras·GDP complex. This helix "loosens up" upon the conformational change to the GTP complex. (It is still recognizable as a helix, but does not have the proper geometry to be an alpha helix.)
At the other end of the "orange group" of amino acids one residue (alanine 58) is "added" to a strand of beta structure when GDP is replaced with GTP. At the same time two residues from the "violet group" (35 and 36) are also converted to beta structure. Taken together these changes cause a lengthening of a section of beta sheet when the ras protein switches from the GDP complex to the GTP complex.
| Copyright © 2000, 2007 F.R. Gorga | Last update: 09-Mar-2007 |