![]() Introduction
to Protein Structure
Introduction
to Protein Structure
Review of Acid-Base Chemistry
Understanding acid-base equlibria is critical to understanding many topics in biochemistry, including peptide/protein chemistry. Thus, we review this topic here.
The overall (or net) charge on a peptide (or protein) depends on its amino acid content and, of course, the pH of the solution in which the peptide resides. Simply, the net charge exhibited by a peptide is the sum of the individual charges present on the amino terminus, the carboxy terminus and any ionizable side chains that are present.
The guiding principle in determining the ionization state of an ionizable group is to remember that when the pH of a solution equals the pKa for an ionizable group, the group exists as a 50:50 mixture of its acidic form and the conjugate base. Also, the further the pH of the solution is from the pKa the more the farther the balance between acid and conjugate base is tipped. If the pH is less than the pKa, then the acid form of the compound predominates. If the pH is greater than the pKa, then the conjugate base predominates. This is shown graphically here:
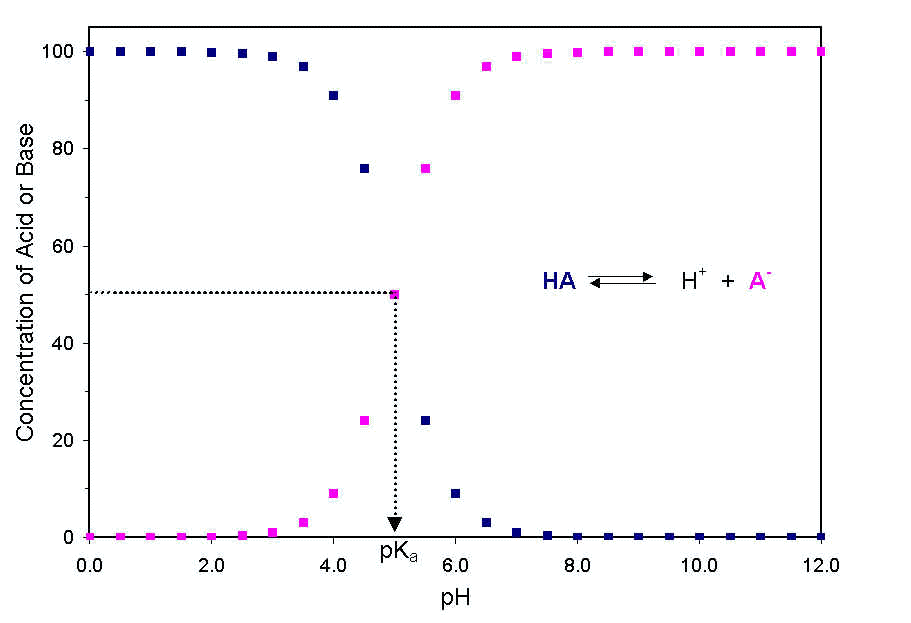
Most ionizable groups fall into two patterns depending on the charge found on the acidic form.
Type I groups are neutral in their acidic form
(HA); they dissociate to form a proton (H+) and an conjugate base (A-)
that is negatively charged: ![]() .
.
Thus, type I groups carry a negative charge when the pH > pKa. Type I groups are neutral when pH < pKa. Examples of type I groups found in proteins include carboxyl groups (the side chains of aspartate and glutamate, as well as the C-terminus), the sulfhydryl group in the side chain of cysteine and the phenolic side chain of tyrosine.
The acid form (HA+) of type II groups
are positively charged and dissociate to form a proton (H+) and an uncharged
conjugate base (A): ![]() .
.
Thus type II groups are uncharged when pH > pKa and carry a positive charge when the pH < pKa. Examples of type II groups found in proteins include amino groups (the side chain of lysine and the alpha-amino group of the N-terminus), the imidazole side chain of histidine, and the guanidinium group of arginine.
Copyright © 1998, 1999, 2007 by Frank R. Gorga; Page maintained by F.R. Gorga; Last updated: 12-Mar-2007