![]() Introduction
to Protein Structure
Introduction
to Protein Structure
Overall Charge on a Peptide -- Example 2
Serylaspartylvalinylglutaminyllysine
(Ser-Asp-Val-Glu-Lys; SEVQK)
Note: ionizable groups are "boxed" |
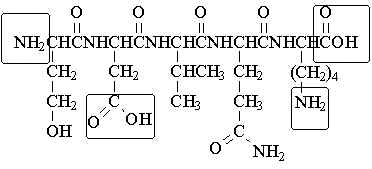 |
In addition to the amino group (pKa ~ 9) and the carboxyl group (pKa ~ 3) at each end of the molecule, the sidechains of the aspartic acid (carboxyl group; pKa ~ 4.4) and the lysine (amino group; pKa ~ 10) residues are ionizable.
Thus, the net charge on this peptide at three different pH's ( 2, 7 and 11) is as follows:
| Charge on Functional Group | ||||||
| pH | N-terminus | Asp -COOH | Lys -NH2 | C-terminus | Net Charge | |
| 2.0 | 1+ | 0 | 1+ | 0 | 2+ | |
| 7.0 | 1+ | 1- | 1+ | 1- | 0 | |
| 11.0 | 0 | 1- | 0 | 1- | 2- | |
Copyright © 1998, 1999, 2007 by Frank R. Gorga; Page maintained by F.R. Gorga; Last updated: 12-Mar-2007