| Drawing Three Dimensional (3-D) Structures on Paper | |
| A Brief Review of Line Structures |
| Line structures are the organic chemists shorthand for drawing chemical structures on paper. | |
| Consider propane, a simple alkane with the molecular formula C3H8. | |
A beginner might draw an extended
structure, showing all of the bonds an atoms in propane: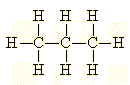 |
|
|
|
|
|
| Thus, chemists have taken to using line structures for most drawing of structures. | |
| The line structure for propane looks like this: |
|
|
|
|
|
| Rules for drawing line structures: | |
|
|
| Look at a few more examples, in order to become familiar with line structures: | |
|
|
| What about stereochemical information or three dimensional information? Please go to the next page for this topic. | |
Copyright © 1996 -1999, 2007 by Frank R. Gorga - All rights reserved.
Last Update: 12-Mar-2007